The Characteristic Bright-line Spectrum of an Atom Is Produced by
The wavelength of the violet Spectrums third line is 4341 nm and so on. Up to 24 cash back The bright-line spectra for three elements and a mixture of elements are shown below.

Hydrogen Spectrum Balmer Series Definition Diagram Spectrum
This emission occurs when an atom element or molecule in an excited state returns to a configuration of lower energy.

. The total number of orbitals that can exist at the 2nd main energy level is A 2 B 4 C 3 D 8 17. If the orbital it jump. 12Explain in terms of both electrons and energy how the bright-line spectrum of an element is produced.
Around the atom. The characteristic bright-line spectrum of an atom is produced when 1 nuclei undergo fission 2 nuclei undergo fusion 3 electrons move from higher to lower energy levels 4 electrons move from lower to higher energy levels. An emission line will appear in a spectrum if the source emits specific wavelengths of radiation.
The characteristic bright-line spectrum of sodium is produced when its electrons A return to lower energy levels B jump to higher energy levels C are lost by neutral atoms D are gained by neutral atoms 16. When electrons jump from the excited state to the ground state the electrons emit energy in the form of light producing a bright-line spectrum. Kirchhoffs Second Law states that a thin hot gas produces an emission line spectrum.
The characteristic bright-line spectrum of an element is produced when electrons A. Move to higher energy levels D. Explanation Verified Reveal next step Reveal all steps Create a free account to see explanations.
The lowest possible energy level for an electron is known as ground state When an electron falls from one energy level to a lower energy level the result is the emission of quantized energy The characteristic bright line spectrum of an element is produced. November 15 2021 Nora FAQ. A likely explanation of this observation would be that an electron moves from a higher to a lower energy level Which is the electron configuration of a 3.
It also explained how light was absorbed and emitted by an atom and why hydrogen and other gases produced their. In 1913 Niels Bohr used the bright line spectrum produced by hydrogen to construct a new model of the atom. 1 2-8-16 2 2-8-14 4 2-8-13 the characteristic bright-line spectrum of an element occurs when electrons move from higher to lower energy levels move from lower to higher energy levels 3 are gained by a neutral atom 4 are lost by a neutral atom when electrons in an atom in an excited state fail to lower energy levels energy is 1.
Fall back to lower energy levels. Quantum theory In quantum mechanics. The diagram shows the characteristic spectral line.
The Balmer series is as follows in the hydrogen emission Spectrum. 11Identify all the elements in the mixture. Emission spectra are produced by thin gases in which the atoms do not experience many collisions because of the low density.
The characteñstic bright-line spectrum oi an atom is produced by its electrons absorbing energy e electrons emitting energy protons absorbing energy 2 protons emitting energy when electrons in an atom in an excited state fall to lower energy levels energy is absorbed only released only neither released nor absorbed both released and. The characteristic bright-line spectrum of an atom is produced by its electrons emitting energy As an atom in the excited state return to the ground state the energy of the atom decreases which subatomic particles have a mass of approximately 1 atomic mass unit each proton and neutron. Are gained from another atom C.
These photons come out as bright lines of a specific wavelength unique to the atom thats producing it on an emission line spectrum. The wavelengths of the lines are characteristic of the element and. H is the red line with the longest wavelength 6563 nm.
Bohrs theory of the atomatoms is known as a line spectrum because the radiation light emitted consists of a series of sharp lines. Are given o as beta particles B. Basically an excited gas in this case neon will emit photons from its excited atoms.
The wavelength of the next line in the blue-green Spectrum is 486. Image will be uploaded soon. The characteristic bright line spectrum of an element is produced when its electrons return to lower energy levels when electrons are in the lowest-energy orbitals available the atom is.
When is the characteristic bright-line spectrum of an atom produced. An electron can jump from one fixed orbital to another. 10State the total number of valence electrons in a cadmium atom in the ground state.
In the laboratory in a flame. The atomic theory proposed by Bohr The characteristic bright-line spectrum of potassium is produced when electrons return to lower energy levels A single burst of light is released from an atom. Bohrs model was the first to explain why if opposite charges attract the electron did not collapse into the nucleus.
Each element has its.
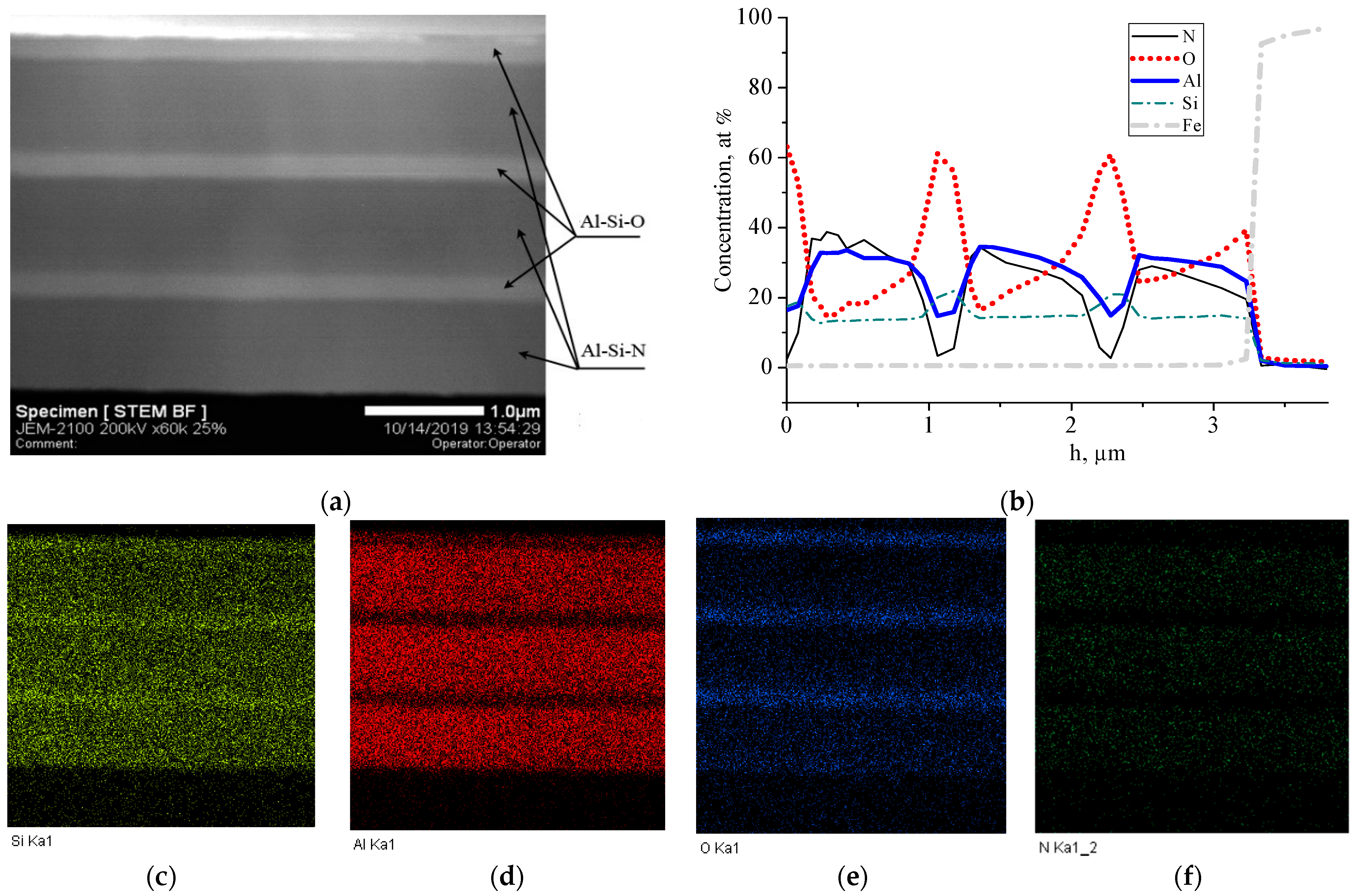
Metals Free Full Text Si Al N O Multi Layer Coatings With Increased Corrosion Resistance Deposited On Stainless Steel By Magnetron Sputtering Html

What Are The States Of Matter Plasma Globe Bowling Balls Bowling Ball

Atomic Regulation Of Metal Organic Framework Derived Carbon Based Single Atom Catalysts For The Electrochemical Co 2 Reduction Reaction Journal Of Materials Chemistry A Rsc Publishing Doi 10 1039 D1ta06915b
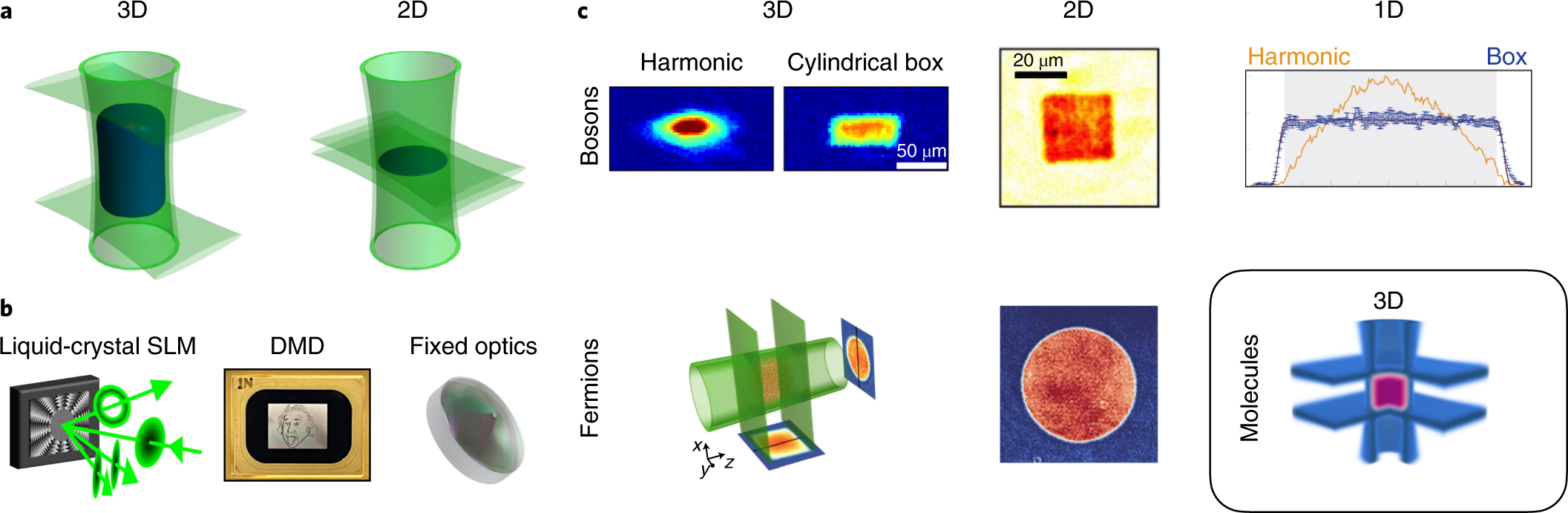
Quantum Gases In Optical Boxes Nature Physics

6 3 Atomic Line Spectra And Niels Bohr Chemistry Libretexts

Opportunities And Challenges In Precise Synthesis Of Transition Metal Single Atom Supported By 2d Materials As Catalysts Toward Oxygen Reduction Reaction Niu 2021 Advanced Functional Materials Wiley Online Library

Experiment 8 Atomic Emission Spectra
How Is The Bright Line Spectrum Of An Element Produced Quora
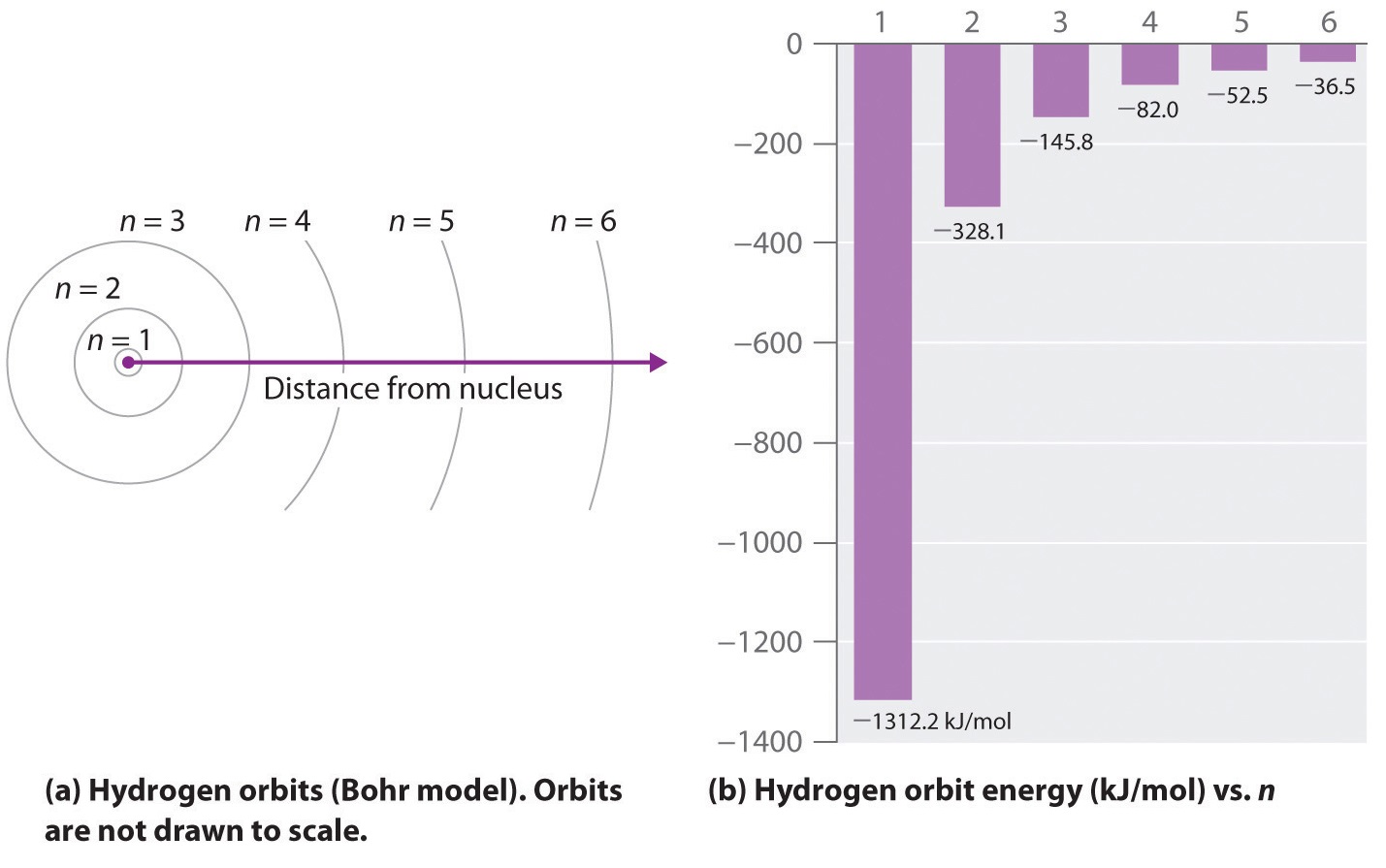
6 3 Line Spectra And The Bohr Model Chemistry Libretexts

Shining Light On Porous Liquids From Fundamentals To Syntheses Applications And Future Challenges Wang 2022 Advanced Functional Materials Wiley Online Library

Light And The Modern Atom Physical Science Earth And Space Science Teaching Chemistry

Enhanced Localized Dipole Of Pt Au Single Site Catalyst For Solar Water Splitting Pnas

Emission Spectrum Definition Types Examples Hydrogen Transitions
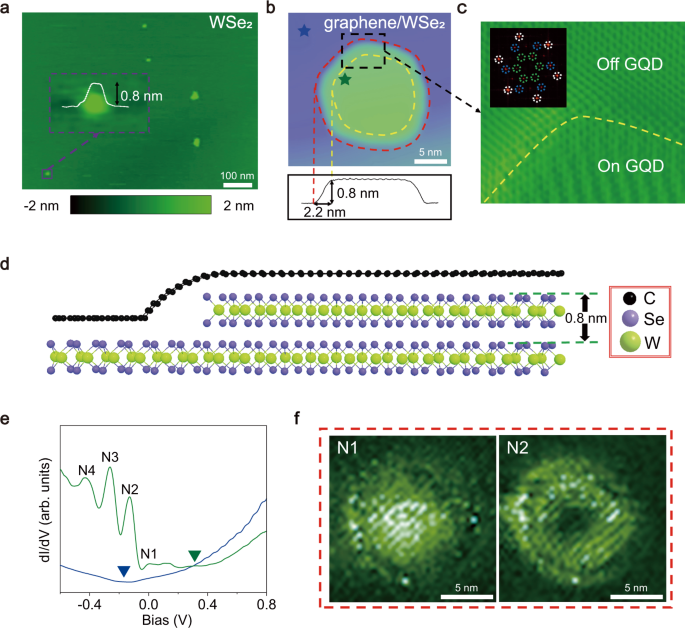
Coexistence Of Electron Whispering Gallery Modes And Atomic Collapse States In Graphene Wse2 Heterostructure Quantum Dots Nature Communications

Homework 6 Spectral Lines Ws Ppt Download


Comments
Post a Comment